FAQs
1. What is unique about cerabone®?
cerabone® is 100% pure bone mineral of bovine origin that is produced by a unique 1200°C manufacturing process1. (BioOss® is heat-treated at ca. 300°C.)
This process ensures maximum safety and leads to an exceptionally high purity of cerabone®, providing ultimate volume stability of the augmented site.
2. What is unique
about the manufacturing process of cerabone®?
The production process of cerabone® utilizes heat (step-wise heating up to >1200°C) and water only (free of chemical additives). (BioOss® is chemically treated and heated at ca. 300°C.)
The sophisticated processing of the bovine bone removes all organic components such as cells and proteins resulting in a 100% pure bone mineral. The exceptionally high purity of cerabone® was confirmed by various analytical methods1.
3. What is the benefit of a 100% pure bone mineral?
Are there differences in the composition between BioOss® and cerabone®?
The exceptionally high purity of cerabone® provides ultimate volume stability, as the particles will only be superficially resorbed.
Due to the unique high temperature treatment process of the bovine bone, cerabone® exhibits a very high crystallinity together with phase purity1. The resorption rate of a bone grafting material decreases as a function of crystal growth during heating – the bigger the crystals, the slower the resorption.
In contrast, BioOss® has a lower crystallinity and in addition, shows a residual amount of 3% water. Its mineral phase also contains carbonate, which is absent in cerabone®. Due to a higher solubility, incorporated carbonate enhances the biodegradation of a grafting material. Therefore, cerabone® may provide a higher volume stability when compared to BioOss®2.
cerabone® particles can be found in the augmentation area even after years, providing long-term volume stability of the grafted site. This has been demonstrated by long-term follow-ups of four to eight years 2-5.
4. Key indications:
What are the advantages of the non-resorbing properties
of cerabone®?
The very slow resorption kinetics of cerabone® make the material the ideal choice in situations where long-term stability is of utmost importance.
In the anterior region where the bony support of the soft tissue is essential to achieve optimal aesthetic results, the long-term stability of cerabone® supports long-term aesthetic outcomes. cerabone® is the ideal biomaterial for preservation of the ridge shape and is specifically indicated to cover and protect autologous or allogenic bone blocks from resorption.
In patients with less adequate bone quality, cerabone® may be preferred because of its high volume stability.
5. What does
the micro- and macroscopic structure
of cerabone® look like?
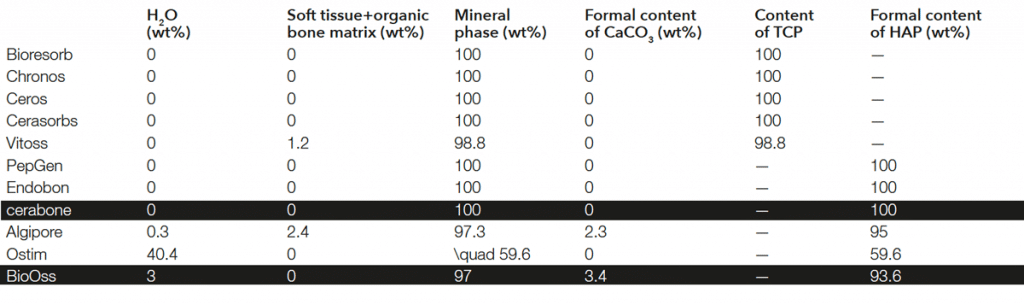
cerabone® has a human-like bone structure6. It is a highly porous bone grafting material*: Macropores enable a fast ingrowth of blood vessels and bone-forming cells, while micropores support quick blood uptake by the capillary effect.
Scanning electron microscopic pictures (SEM) demonstrate the highly structured surface of cerabone®. The rough, hydrophilic surface facilitates the adhesion of signaling proteins and cells from the blood. Adhering osteoblasts can initiate the formation of bone matrix, leading to the osseous integration of the particles.
*Porosity of ~65-80% and a mean pore size of ~600-900 μm7
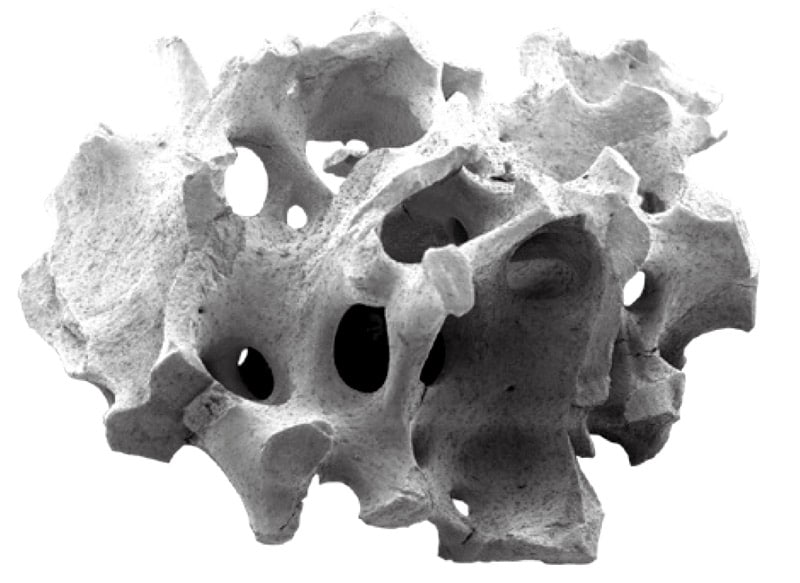
Trabecular (cancellous) bone structure of a cerabone® particle

Electron microscopic close-up showing the rough surface of cerabone®
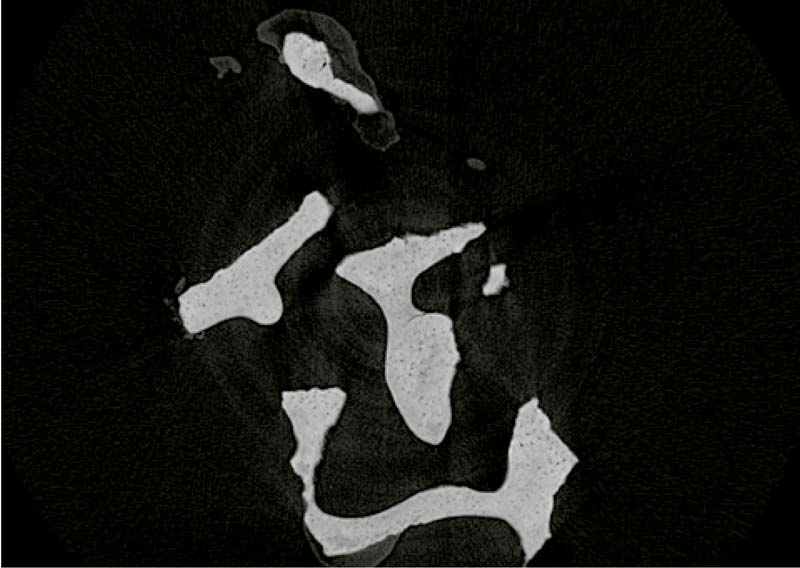
Cross-section of a cerabone® particle (μ-CT) revealing the lamellar structure of cerabone®
6. Is cerabone® hydrophilic?
Why is hydrophilicity an important factor of a bone grafting material?
Yes! The rough surface and three-dimensional pore network of cerabone® lead to an excellent hydrophilicity, which was shown to be superior as compared to other bone grafting materials8.The efficient blood uptake of cerabone® is demonstrated in this video:
https://www.youtube.com/watch?v=oSYYaPMoZBY.
The strong capillary effect enables a fast and efficient penetration with fluids, blood and proteins into the three-dimensional trabecular network of the particles. cerabone® binds and gradually releases signaling molecules from the blood thereby providing a longterm depot-effect.
In addition, following hydration the particles stick together facilitating their application to the defect site (see next question).
7. Is cerabone® sticky?
Yes, the cerabone® particles stick well together after hydration. However, some clinical users that are familiar with non-sintered materials may have the feeling that cerabone® particles are not as „sticky“ as BioOss® particles. This perception may be caused by the smaller particle size of BioOss®, i.e. the bigger range in the size of the small BioOss® granules (0.25 – 1.0 mm for BioOss® compared to 0.5 – 1.0 mm for cerabone®). Mixing small particles with fluids may yield a different consistency compared to large particles.
The size of the cerabone® particles allow to leave sufficient space between the particles for new bone formation. Since cerabone® is non-resorbable, the formation of vital bone between the particles is of utmost importance. Very small particles may block these interparticular spaces.
8. What happens with cerabone®
after implantation?
Due to the human-like bone structure of cerabone®, bone formation starts early following implantation (3-6 weeks).
During the formation of a blood clot, different cell types involved in the wound healing cascade and growth factors originating from the wound area bind to the surface of the scaffold. Precursor cells differentiate into osteoblasts and start to produce new bone matrix.
After a few months (6-9 months), the cerabone® particles are integrated into the newly formed bone matrix and the surface is completely covered by new mineralized bone resulting in a long-term stable bone situation.
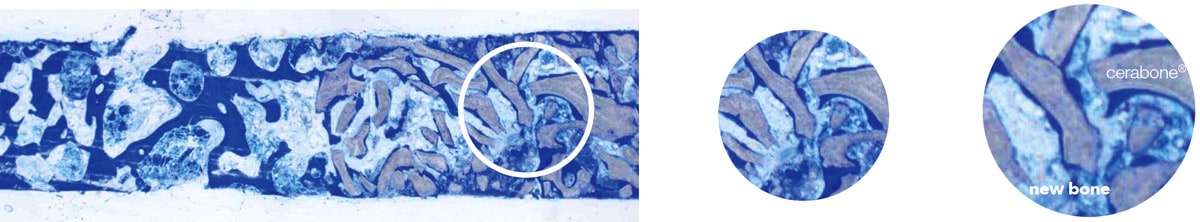
Histology of a trephine biopsy taken six months after sinus floor augmentation with cerabone® (grey) showing the excellent integration of the particles into the newly formed bone (blue).
9. When should the re-entry/implant placement
be performed?
It will take about six months until the particles are completely integrated into the newly formed bone matrix9.
Accordingly, a healing phase of at least six months should be kept to ensure a stable integration of the particles. For smaller defects the healing time can be shorter.
Likewise, an earlier re-entry is possible, i.e. after four months, if cerabone® is mixed with autologous or allogenic bone.
10. For which indications
are the two particles sizes recommended?
The small particles are particularly advantageous for contouring, e.g. for augmentation in the aesthetic region or to fill remaining gaps when a block grafting
is performed. Small particles are also preferably used for the regeneration of smaller defects and intraosseous defects.
Large particles are favorable if large volume defects (e.g. sinus floor elevation) are filled. In addition to the higher volume, there is more space between the large particles, which enables a better revascularization of bigger defects.
11. How can I compare
the volume of cerabone®
to the weight of BioOss®?
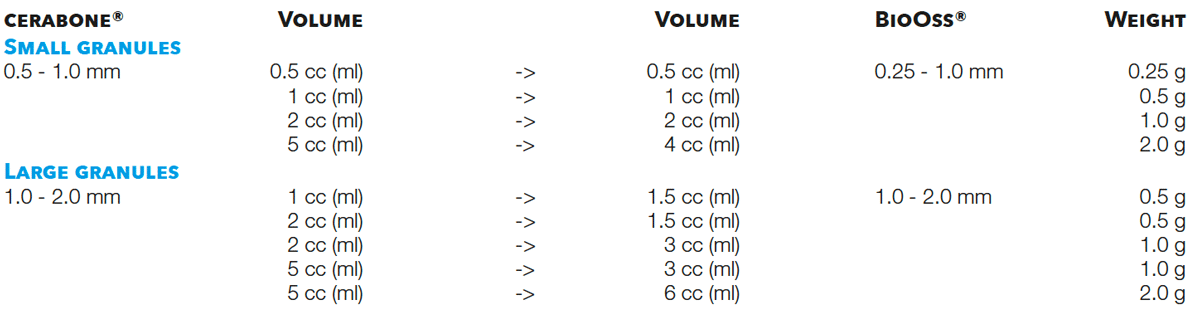
To determine the required amount of a grafting material it is important to estimate the size of the defect and the volume needed to fill that defect. Accordingly, the volume cc (ml) should be compared (instead of the weight (g) of a material).
The smallest package of cerabone® (0.5 cc (ml)) corresponds to the smallest package of BioOss® (0.25 g) as this has a volume of 0.5 cc (ml).
For the large particles, there is no direct equivalent for cerabone® as 0.5 g of the large BioOss® particles (1.0 – 2.0 mm) have a volume of 1.5 cc (ml). You can either chose 1 cc (ml) or 2 cc (ml) of cerabone®.
The volumes of the BioOss® packages can also be found on the Geistlich price list.
12. What if
cerabone® particles appear in the soft tissue?
If the augmented site (or filled extraction socket) was not or only incompletely covered with a membrane or if the membrane has resorbed too quickly due to exposure, particles in the mucosa can sometimes be observed.
However, it can also happen that particles are visible in the soft tissue if the augmentation site was covered adequately. Particles in the soft tissue pose no risk to the patient and do not adversely affect the healing process, as their appearance is not associated with an inflammation.
– Particles visible in the superficial part of the mucosa will grow out.
– Particles at the inner aspect of the flap, visible at time of re-entry, can be removed with forceps. Also, loose particles in the most coronal part of the augmentation site that are not embedded in bone matrix can be scratched away.
To avoid a migration of particles it is of utmost importance to stabilize the grafting area by covering with a membrane. A fixation of the membrane (e.g. with pins) can be advantageous. Furthermore, mixing of the particles with PRF or hyaluronic acid may also help to stabilize the particles.
13. Is cerabone® a safe product?
Yes! Because of the choice of the raw material (cattle destined for food industry) and the unique manufacturing process (heating up to >1200°C), cerabone® can be considered a maximum safe product:
– The heating process removes all organic components, including potential bacteria, viruses and prions.
– Heating above 800°C ensures a complete inactivation of the infectivity of potential prions10-13.
– The countries of origin of the cattle used for the manufacturing of cerabone® are “recognized as having a negligible BSE risk” according to the World Organization for Animal Health (OIE Resolution No. 20, 27 May 2016).
– Moreover, muscle and skeletal tissue of cows is classified as tissue without demonstrated risk of BSE-infectivity or prions (PrPTSE) by the WHO (WHO Tables in
Tissue Infectivity Distribution in Transmissible Spongiform Encephalopathies, 2010).
cerabone® and its production processes fulfill all guidelines and safety regulations of Germany and the EU for bovine bone grafting material including DIN EN ISO 22442-1, DIN EN ISO 22442-2 and DIN EN ISO 22442-3. The end product is gamma-sterilized and stored in a double-sterile barrier packaging.
14. Could a patient’s inflammatory reaction (swelling, redness)
be caused by an allergic reaction against the material?
cerabone® is 100% pure bone mineral as all organic components including antigenic components are removed during the high temperature treatment process1.
Allergic reactions can be virtually excluded. The product is supplied sterile.
From our experience, most problems appear because of allergic reactions against antibiotics or because of contaminations of the material with saliva prior to implantation.
Nonetheless, in case of complications customers are encouraged to lodge a complaint.
The complaint must include the product code and lot number of the applied material(s), a description of the issue and the problems occurred.
15. For how long has cerabone® been used
in regenerative medicine?
cerabone® has been on the market since 2002 and is distributed in about 100 countries. It has been successfully applied in more than 1 Million patients
in regenerative dentistry and has been in use for more than 15 years in various medical applications (e.g. craniofacial surgery, oncology and handand
spine surgery).
REFERENCES:
1. Tadic, D. and Epple, M. (2004), “A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to
natural bone”, Biomaterials, Vol. 25 No. 6, pp. 987–994.
2. Riachi, F., Naaman, N., Tabarani, C., Aboelsaad, N., Aboushelib, M.N., Berberi, A. and Salameh, Z. (2012), “Influence of material properties on rate of
resorption of two bone graft materials after sinus lift using radio graphic assessment”, International journal of dentistry, Vol. 2012, p. 737262.
3. Lorean A, Mazor Z, Barbu H, Mijiritsky E, Levin L (2014) „Nasal Floor Elevation Combined with Dental Implant Placement: A Long-Term Report of up to
86 Months“, Int J Oral Maxillofac Implants 29 (3), 705-708. May-Jun 2014.
4. Tawil G, Barbeck M, Unger R, Tawil P, Witte F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute
as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int J Oral Maxillofac Implants. 2018 September/October;33(5):1089–1096.
5. Tawil G, Tawil P, Khairallah A. Sinus Floor Elevation Using the Lateral Approach and Bone Window RepositioningI: Clinical and Radiographic Results in 102
Consecutively Treated Patients Followed from 1 to 5 Years. Int J Oral Maxillofac Implants. 2016 Jul-Aug;31(4):827-34.
6. Tadic, D., Beckmann F., Donath T and Epple M. Comparison of different methods for the preparation of porous bone substitution materials and structural
investigations by synchrotron μ-computer tomography. Mat.-wiss. u. Werkstofftech. 2004, 35, No. 4.
7. Seidel, P. and Dingeldein, E. (2004), “Cerabone®– eine Spongiosa-Keramik bovinen Ursprungs”, Materialwissenschaft und Werkstofftechnik,
Vol. 35 No. 4, pp. 208–212.
8. Trajkovski B., Jaunic, M., Müller W.H., Beuer F., Zafiropoulos G.-G. and Houshmand A. Hydrophilicity, Viscoelastic, and Physico chemical Properties
Variations in Dental Bone Grafting Substitutes. Materials 2018, 11(2), 215.
9. Rothamel D, Schwarz F, Fienitz T, Smeets R, Dreiseidler T, Ritter L, Happe A, Zöller J. Biocompatibility and biodegradation of a native porcine pericardium
membrane: results of in vitro and in vivo examinations. Int J Oral Maxillofac Implants. 2012 Jan-Feb;27(1):146-54.
10. Becker, Organikum, Ambrosius Verlag, Leipzig 1993.
11. Morrison, Boyd, VCH 1986.
12. Murugan R, Panduranga Rao K, Sampath Kumar T S. Heat-deproteinated xenogeneic bone from slaughterhouse waste: Physico-chemical properties.
Bulletin of Materials Science 2003;26(5):523-528.
13. Brown, P., Rau, E.H., Johnson, B.K., Bacote, A.E., Gibbs, C.J. and Gajdusek, D.C. (2000), “New studies on the heat resistance of hamster-adapted scrapie
agent: threshold survival after ashing at 600 degrees C suggests an inorganic template of replication”, Proceedings of the National Academy of Sciences of
the United States of America, Vol. 97 No. 7, pp. 3418–3421.